
![]()
![]()
1,2,3 Cecilija Rotim
1,4 Biljana Filipović
1,4 Snježana Čukljek
1,5 Mara Županić
1,6 Damir Važanić
7 Danijela Kundrata
1 University of Applied Health Sciences, Zagreb, Croatia
2 Faculty of Dental Medicine and Health Osijek,
Josip Juraj Strossmayer University of Osijek, Osijek, Croatia
3 Rotim Polyclinic, Zagreb, Croatia
4 Faculty of Health Studies, University of Rijeka, Rijeka, Croatia
5 Department of Nursing, University of Dubrovnik, Dubrovnik, Croatia
6 Croatian Institute for Emergency Medicine, Zagreb, Croatia
7 General Hospital “Dr. Ivo Pedišić”, Sisak, Croatia
Article received: 22.10.2024.
Article accepted: 06.12.2024.
https://doi.org/10.24141/2/8/2/10
Author for correspondence:
Cecilija Rotim
Rotim Polyclinic, Miramarska 24, 10000 Zagreb, Croatia e-mail: cecilija.rotim@rotim.hr
![]()
Keywords: celiac disease, gluten-free diet, transglutaminases
![]()
![]()
Introduction. Celiac disease (CD) is an autoimmune disorder triggered by gluten ingestion in genetically predisposed individuals. Gluten immunogenic pep- tides (GIP) in feces and tissue transglutaminase (tTG) are key biomarkers for monitoring gluten intake and immune response, respectively. Despite the increas- ing use of GIP for assessing gluten-free diet (GFD) adherence, its correlation with tTG remains unclear. Understanding their relationship could enhance CD monitoring.
Aim. To evaluate fecal GIP concentrations in CD pa- tients, examine their correlation with tTG levels, and assess the utility of combining these biomarkers for CD management.
Methods. This cross-sectional study included 60 CD patients adhering to a GFD and 10 healthy controls. Fecal and serum GIP levels were quantified using ELISA tests, and tTG concentrations were measured. Statistical analyses included Mann-Whitney U tests for group comparisons and Spearman’s rank correla- tion for assessing relationships between biomarkers.
Results. Median fecal GIP concentration in CD pa- tients was significantly lower (39.0 ng/g) compared to controls (474.2 ng/g; p<0.001), confirming GFD adherence. Similarly, serum GIP was lower in the CD group (p<0.001). No significant correlation was found between GIP and tTG levels (Rho=0.114, p=0.387), indicating they measure distinct aspects of CD activity.
![]()
Conclusion. This study specifically evaluated fecal GIP concentrations in patients with celiac disease and their correlation with tTG levels. Our findings in- dicate no significant correlation, demonstrating that these biomarkers assess different aspects of disease activity. This study confirms the sensitive nature of GIP for detecting gluten intake and tTG’s role in reflecting immune response and mucosal damage. Hence, the integrated use of these biomarkers, as suggested by our results, can improve the manage- ment and monitoring of celiac disease, providing a more precise assessment of dietary adherence and immune activity.
![]()
![]()
Celiac disease (CD) is a chronic autoimmune disorder triggered by the ingestion of gluten - a protein found in wheat, rye, and barley. The disease occurs in ge- netically predisposed individuals and is mediated by tissue transglutaminase (tTG), a ubiquitous enzyme that serves as the primary autoantigen in CD. The re- sulting immune response leads to structural damage in the small intestine, characterized by villous atro- phy, crypt hyperplasia, and infiltration of intraepithe- lial lymphocytes, disrupting nutrient absorption and contributing to a range of gastrointestinal and sys- temic symptoms (1,2).
The prevalence of CD has increased significantly over recent decades and is now estimated at 1–2% global- ly (3). This rise is largely attributed to enhanced diag- nostic capabilities, including the availability of highly sensitive and specific serological tests such as those detecting tTG antibodies. Improved screening has also facilitated the identification of subclinical cases, even among elderly populations (4). Environmental and dietary factors, including increased gluten con- sumption (up to 20 g/day in certain populations) and changes in gluten quality due to agricultural inno- vations, are thought to contribute to this trend (5). Moreover, the “hygiene hypothesis” suggests that reduced exposure to pathogens in industrialized so- cieties has led to a dysregulated immune response, further increasing the prevalence of autoimmune conditions like CD (6).
The mainstay of CD management is strict, lifelong ad- herence to a gluten-free diet (GFD), which alleviates symptoms, promotes intestinal healing, and reduces the risk of complications such as refractory CD and small intestinal lymphoma (7). However, maintaining adherence remains challenging due to the ubiquitous presence of gluten in processed foods and the social and economic burdens associated with dietary restric- tions. The availability of gluten-free products has in- creased dramatically over the past five years, with such products now widely accessible in major supermarkets, health food stores, and online retailers (8). Nonethe- less, these products remain significantly more expen- sive than their gluten-containing counterparts (9,10).
The assessment of GFD compliance traditionally re- lies on self-reports, dietary interviews, serological tests (e.g., anti-tTG antibodies), or small bowel biop- sies. However, these methods have significant limita- tions. Self-reports are often unreliable due to inten- tional or unintentional inaccuracies, while serological markers correlate poorly with mucosal healing (10). Small bowel biopsies, although the gold standard for assessing mucosal recovery, are invasive and not routinely performed, particularly in asymptomatic pa- tients who show clinical improvement (11,12).
Gluten immunogenic peptides (GIP) are a promising new biomarker for monitoring dietary adherence. These peptides, derived from immunotoxic frag- ments of gluten such as the α-gliadin-33-mer, are resistant to enzymatic digestion and are excreted in- tact in stool or urine. Their detection directly reflects gluten ingestion, providing an objective measure of dietary transgressions (13,14).
Research has shown that fecal GIP concentrations can remain detectable for up to four days after glu- ten ingestion, making them a highly sensitive indica- tor of recent dietary lapses (13). For example, one study demonstrated that 30% of CD patients on a GFD for at least one year had detectable fecal GIP, indicating dietary noncompliance. In comparison, se- rological tests identified dietary infractions in only 18% of patients, underscoring the superior sensitiv- ity of GIP detection (14).
Despite these advances, the relationship between fecal GIP concentrations and traditional markers of CD activity, such as tTG, remains unclear. Investigat- ing this correlation is critical to understanding how GIP testing can complement existing diagnostic tools in CD management.
![]()
![]()
This study aims to:
Assess fecal GIP concentrations in CD patients.
Analyze the relationship between fecal GIP levels and tTG concentrations.
![]()
![]()
This observational, cross-sectional study was con- ducted at the Clinical Nutrition Outpatient Depart- ment of the Clinical Hospital Center Zagreb over a 12-month period. The study involved a total of 70 participants, including 60 adult patients diagnosed with celiac disease and a control group of 10 healthy volunteers who eat food containing gluten.
The inclusion criteria required participants to be over 18 years of age, have a confirmed diagnosis of ce- liac disease, and provide written informed consent. Exclusion criteria encompassed individuals under 18 years of age, as well as those diagnosed with gas- trointestinal diseases such as diverticulitis, entero- colitis, or ischemic colitis. Additionally, patients with liver dysfunction (e.g., cirrhosis or active hepatitis), chronic kidney disease, severe hypertension, coro- nary artery disease, or peripheral arterial disease were excluded. Other exclusion criteria included he- matological, malignant, or autoimmune disorders, as well as pregnancy.
The study protocol was approved by the Ethics Com- mittee of the Clinical Hospital Center Zagreb (Ap- proval No. 02/21 AG, Class: 8.1-19/153-2). All par- ticipants provided written informed consent prior to enrolment, and the study was conducted in accord- ance with the principles of the Declaration of Hel- sinki.
Data were presented in tables and figures. The nor- mality of data distribution was evaluated using the Kolmogorov-Smirnov test, which revealed that most continuous variables did not follow a normal distri- bution. As a result, nonparametric tests were used in further analyses. Differences between continuous variables in independent groups were analyzed us- ing the Mann-Whitney U test, and significant results were displayed with Box-and-Whisker plots. Fisher’s exact test was employed for categorical data. Spear- man’s rank correlation coefficient (Rho) was used for correlational analyses.
A significance level of p<0.05 was used for all analy- ses. Statistical analyses were performed using Med- Calc® Statistical Software version 20.022 (MedCalc Software Ltd, Ostend, Belgium; https://www.med- calc.org; 2021).
Power analysis for Fisher’s exact test was based on pilot study results, estimating a 100% positive GIP biomarker detection rate among non-compliant par- ticipants and a 30% detection rate among compliant participants. For a power of 90% and a significance level (α) of 0.05, with a case-to-control ratio of 6:1, a minimum of 35 participants was required (30 in the study group and 5 in the control group). Power analy- sis was performed using MedCalc® Statistical Soft- ware version 20.022.
Demographic and anthropometric data were gath- ered through a structured questionnaire, medical re- cords, and direct measurements. Blood samples were analyzed for complete blood count and routine bio- chemical parameters. Fecal and urinary gluten immu- nogenic peptides were detected using the iVYLISA GIP ELISA test (Biomedal SL).
![]()
![]()
Descriptive statistics of anthropometric indicators for the included celiac disease patients (N=60) are pre- sented in Table 1.
Table 1. Descriptive statistics of anthropometric indicators for the included celiac disease patients (N=60) | |||||||
Arithmetic Mean | Standard Deviation | Min | Max | Centile | |||
25. | Median | 75. | |||||
Age | 45.52 | 12.91 | 19.00 | 77.00 | 3725 | 44.00 | 55.00 |
Body Mass (kg) | 67.68 | 15.34 | 45.60 | 128.50 | 57.55 | 62.70 | 75.80 |
Height (cm) | 168.56 | 8.93 | 143.00 | 188.00 | 163.00 | 169.00 | 174.00 |
Body Mass Index (BMI) (kg/m²) | 23.73 | 4.40 | 18.10 | 37.80 | 20.80 | 22.60 | 25.75 |
Lean Mass (kg) | 47.74 | 9.86 | 37.30 | 82.90 | 41.85 | 44.50 | 49.50 |
Muscle Mass (kg) | 45.32 | 9.39 | 35.40 | 78.80 | 39.75 | 42.20 | 47.00 |
Bone Mass (kg) | 2.42 | 0.47 | 1.90 | 4.10 | 2.10 | 2.30 | 2.50 |
Basal Metabolic Rate (BMR) (kcal) | 1429.37 | 295.14 | 1111.00 | 2556.00 | 1260.00 | 1367.00 | 1458.00 |
Visceral Fat Index | 5.54 | 3.70 | 1.00 | 18.00 | 3.00 | 5.00 | 7.00 |
The median Body Mass Index (BMI) of our study par- ticipants, which was recorded at 22.6 kg/m² with an interquartile range of 20.8 to 25.8, classifies them within the “Normal Weight” category according to the World Health Organization standards. This categoriza- tion is defined for BMIs ranging from 18.5 to 24.9 kg/ m² (15). The median muscle mass of the participants was 42.2 kg (39.8–47.0), which is within the range for healthy adults, considering variations due to age, gen- der, and body size. Similarly, the median basal meta- bolic rate (BMR) of 1367.0 kcal (1260.0–1458.0) is in line with expected values for a population of similar demographic characteristics, factoring in the influenc- es of age, sex, and muscle mass. Both measurements indicate a healthy physiological status among the participants, similar to the median visceral fat index value, which was within the acceptable range (<13) and amounted to 5.0 (3.0–7.0). These results shows that physical condition of the participants does not exhibit deviations that would likely impact the study’s outcomes related to celiac disease biomarkers.
Descriptive statistics of biomarkers in celiac dis- ease patients (N=60) are presented in Table 2 and Figures 1 - 3. The median serum GIP concentration
was 0.78 (0.78–0.78) /mL, while the median fe- cal GIP concentration was 39.0 (39.0–39.0) /g. The median tissue transglutaminase (tTg) concentration was 5.20 (0.85–14.70). Serum GIP values showed a perfect correlation with fecal GIP values (Rho=1.000, p<0.001), allowing these biomarker concentrations to be treated as a single variable.
Table 3 presents an analysis of differences in the dis- tribution of GIP biomarker results between the study and control groups, treated as categorical variables (positive vs. negative findings). Positive results were defined as any detectable GIP concentrations using the applied analytical method. The control group, consisting of participants not adhering to a gluten- free diet, exhibited a significantly higher frequency of positive results.
These analyses reveal significantly higher GIP bio- marker concentrations in participants from the control group, who were not on a gluten-free diet (p<0.001). This finding supports the conclusion that the study group, comprising celiac disease patients, adhered to a gluten-free dietary regimen, unlike the control group (Table 4).
Table 2. Descriptive statistics of biomarkers in celiac disease patients (N=60) | |||||||
Arithmetic Mean | Standard Deviation | Min | Max | Centile | |||
25. | Median | 75. | |||||
GIP/mL (dil 10) | 1.74 | 2.27 | 0.78 | 9.95 | 0.78 | 0.78 | 0.78 |
GIP/g in stool | 86.96 | 113.43 | 39.00 | 497.70 | 39.00 | 39.00 | 39.00 |
tTg (IgA) | 111.12 | 634.52 | 0.85 | 4861.10 | 0.85 | 5.20 | 14.70 |
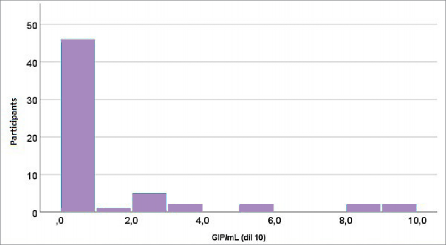
Figure 1. Distribution of measured GIP concentrations (GIP/mL, dilution 10) in the study group (celiac disease patients)
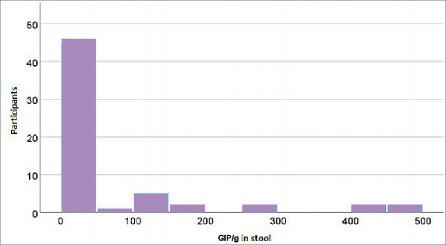
Figure 2. Distribution of measured GIP concentrations (GIP/g) in fecal samples from the study group (celiac disease patients)
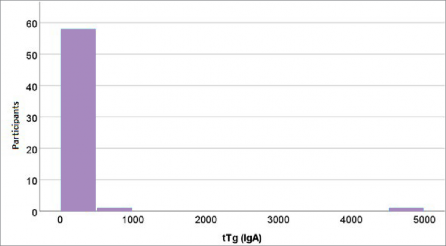
Figure 3. Distribution of measured tissue transglutaminase concentrations in the study group (celiac disease patients)
Table 3. Analysis of differences in GIP biomarker distribution between the study and control groups: Fisher’s exact test | ||||||
Groups | ||||||
Study group | Control | p | ||||
N | % | N | % | |||
GIP / mL (dil 10) categories | Normal Level | 47 | 78.3 | 0 | 0 | <0.001 |
Elevated | 13 | 21.7 | 10 | 100 | ||
GIP / g in stool categories | Normal Level | 47 | 78.3 | 0 | 0 | <0.001 |
Elevated | 13 | 21.7 | 10 | 100 | ||
Table 4. Analysis of differences in GIP serum and GIP fecal concentrations between the study and control groups | |||||||||
Centile | |||||||||
Groups | Min | Max | 25. | Median | 75. | Mann- Whitney U | Z | p | |
GIP / mL (dil 10) | Study group | 0.78 | 9.95 | 0.78 | 0.78 | 0.78 | |||
Control | 4.39 | 14.22 | 7.80 | 9.49 | 13.16 | ||||
21.000 | -5.533 | <0.001 | |||||||
Study group | 39.00 | 497.70 | 39.00 | 39.00 | 39.00 | ||||
GIP / g in stool | |||||||||
Control | 219.43 | 711.23 | 390.38 | 474.20 | 658.32 | ||||
The correlation between GIP biomarker concentra- tions and tissue transglutaminase levels using Spear- man’s rank correlation coefficient (Rho) indicate no significant correlation between GIP concentrations and tissue transglutaminase (Rho=0.114, p=0.387).
![]()
![]()
The results of this study demonstrate that the me- dian fecal concentrations of gluten immunogenic peptides in celiac disease patients were significantly lower compared to the control group not adhering to a gluten-free diet (Table 5). Similarly, serum GIP concentrations were also significantly lower in the study group (Table 4), confirming the adherence of the study group to the gluten-free diet. These findings underscore the high sensitivity of GIP as a biomarker for detecting gluten intake in individuals with CD. Previous studies have confirmed that GIP is a reliable tool for quantitative monitoring of dietary infractions, even with occasional gluten exposure,
surpassing traditional monitoring methods such as serological tests (14-17).
The association between fecal GIP concentrations and tissue transglutaminase was examined using Spear- man’s rank correlation coefficient (Table 6). The results indicated no significant correlation between these two biomarkers, suggesting that GIP and tTG measure dif- ferent aspects of disease activity. GIP directly reflects gluten intake, while tTG represents the immune re- sponse and potential mucosal damage. These findings are in line with prior studies showing that tTG does not reflect occasional dietary infractions, whereas GIP enables precise detection of actual gluten ingestion, including its effects on intestinal mucosa (13,18,19). Furthermore, studies have confirmed a significant as- sociation between fecal GIP detection and future his- tological changes, highlighting GIP as an extremely valuable biomarker in clinical practice (14,17).
The graphical representation of GIP concentration distributions in serum and stool (Figures 1 and 2) fur- ther supports these findings, showing highly uniform values in patients adhering to the GFD and wide vari- ability in the control group. This uniformity among adherent patients corroborates findings demonstrat- ing the high specificity of GIP in detecting gluten in-
take, as consistently observed in studies across vari- ous age groups (14,17). For example, research has shown that younger children (<3 years old), under strict parental dietary supervision, exhibit lower GIP levels compared to adolescents, where dietary non- adherence is more common. Conversely, the distribu- tion of tTG (Figure 3) reveals significant variability in this marker among patients but no clear association with GIP concentrations. This confirms that tTG bet- ter reflects long-term immune activation, while GIP accurately measures recent gluten exposure (16).
These results highlight the potential complementa- rity of GIP and tTG in CD monitoring. GIP offers an immediate assessment of gluten intake, while tTG, particularly when combined with other indicators, aids in evaluating immune responses and chronic dis- ease activity. The combination of these two biomark- ers could enable more accurate and comprehensive monitoring of CD patients, as supported by studies emphasizing the benefits of integrating both meth- ods into clinical practice (17,19).
Further studies with larger and more diverse cohorts are needed to validate these findings and establish standardized thresholds for GIP and tTG concen- trations in clinical settings. Longitudinal research should also explore the relationship between GIP lev- els, tTG concentrations, and clinical outcomes, such as symptom severity and mucosal healing, to refine their combined use in CD monitoring.
This study has several limitations. The sample size may restrict the generalizability of the findings, par- ticularly when evaluating the variability of GIP and tTG biomarkers across different subgroups. Addition- ally, while GIP is highly sensitive to recent gluten intake, its short detection window may not capture long-term dietary adherence, unlike tTG, which re- flects cumulative immune response. This temporal difference may explain the lack of significant corre- lation between the two biomarkers. Variability in di- etary habits, including inadvertent gluten consump- tion, could have influenced GIP levels and affected the interpretation of results.
![]()
![]()
This study rigorously evaluated fecal GIP concentra- tions in celiac disease patients and examined their correlation with serum tTG levels. Our findings reveal no significant correlation between these biomarkers, indicating they assess different dimensions of celiac disease pathology. This study confirms GIP’s efficacy in detecting gluten intake and tTG’s capacity to re- flect immune response and mucosal damage. Inte- grating these biomarkers can thus enhance the accu- racy of dietary compliance assessments and improve the monitoring of immune activity in the manage- ment of celiac disease. These findings suggest the potential for developing more nuanced strategies that utilize both biomarkers to tailor patient manage- ment more effectively.
![]()
![]()
![]()
Volta U, Caio G, Stanghellini V, De Giorgio R. The chan- ging clinical profile of celiac disease: a 15-year expe- rience (1998-2012) in an Italian referral center. BMC Gastroenterol. 2014;14:194.
Sharma N, Bhatia S, Chunduri V, Kaur S, Sharma S, Ka- poor P, et al. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front Nutr. 2020;7:6.
de Lorgeril M, Salen P. Gluten and wheat intolerance today: are modern wheat strains involved? Int J Food Sci Nutr. 2014;65(5):577–81.
Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol. 2018;18(2):105–20.
Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, Ca- tassi C, et al. Celiac disease: a comprehensive current review. BMC Med. 2019;17(1):142.
Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367(25):2419-26.
Rostami K, Bold J, Parr A, Johnson MW. Gluten-Free Di- et Indications, Safety, Quality, Labels, and Challenges. Nutrients. 2017;9(8):846.
Manufacturers and Retailers [Internet]. Coeliac UK. Available from: https://www.coeliac.org.uk/food-in- dustry-professionals/manufacturers-and-retailers Ac- cessed: 01.05.2024.
Lee AR, Wolf RL, Lebwohl B, Ciaccio EJ, Green PHR. Persistent Economic Burden of the Gluten Free Diet. Nutrients. 2019;11(2):399.
Panagiotou S, Kontogianni MD. The economic bur- den of gluten-free products and gluten-free diet: a cost estimation analysis in Greece. J Hum Nutr Diet. 2017;30(6):746-52.
Bannister EG, Cameron DJ, Ng J, Chow CW, Oliver MR, Alex G, et al. Can celiac serology alone be used as a marker of duodenal mucosal recovery in children with celiac disease on a gluten-free diet? Am J Gastroen- terol. 2014;109(9):1478-83.
Volta U, Fabbri A, Parisi C, Piscaglia M, Caio G, Tovoli F, et al. Old and new serological tests for celiac dis- ease screening. Expert Rev Gastroenterol Hepatol. 2010;4(1):31-5.
Comino I, Real A, Vivas S, Síglez MÁ, Caminero A, Nistal E, et al. Monitoring of gluten-free diet com- pliance in celiac patients by assessment of gliadin 33-mer equivalent epitopes in feces. Am J Clin Nutr. 2012;95(3):670-7.
Comino I, Fernández-Bañares F, Esteve M, Ortigosa L, Castillejo G, Fambuena B, et al. Fecal Gluten Pep- tides Reveal Limitations of Serological Tests and Food Questionnaires for Monitoring Gluten-Free Diet in Celiac Disease Patients. Am J Gastroenterol. 2016;111(10):1456-65.
World Health Organization. Body Mass Index (BMI). Geneva: World Health Organization. Available from: https://who-sandbox.squiz.cloud/en/health-topics/di- sease-prevention/nutrition/a-healthy-lifestyle/body- mass-index-bmi Accessed: 5.12.2024.
Coto L, Mendia I, Sousa C, Bai JC, Cebolla A. Deter- mination of gluten immunogenic peptides for the management of the treatment adherence of celiac disease: A systematic review. World J Gastroenterol. 2021;27(37):6306-21.
Porcelli B, Ferretti F, Biviano I, Santini A, Cinci F, Vas- cotto M, et al. Testing for fecal gluten immunoge- nic peptides: a useful tool to evaluate compliance with gluten-free diet by celiacs. Ann Gastroenterol. 2020;33(6):631-7.
Wild D, Robins GG, Burley VJ, Howdle PD. Evidence of high sugar intake, and low fibre and mineral inta- ke, in the gluten-free diet. Aliment Pharmacol Ther. 2010;32(4):573-81.
Kumar V, Jarzabek-Chorzelska M, Sulej J, Karnewska K, Farrell T, Jablonska S. Celiac disease and immuno- globulin a deficiency: how effective are the serolo- gical methods of diagnosis? Clin Diagn Lab Immunol. 2002;9(6):1295-300.
![]()
![]()
![]()
![]()
Uvod. Celijakija je autoimuni poremećaj uzrokovan unosom glutena kod genski predisponiranih osoba. Glutenski imunogeni peptidi (GIP) u stolici i tkivna transglutaminaza (tTG) ključni su biomarkeri za pra- ćenje unosa glutena i imunosnog odgovora. Unatoč sve češćoj primjeni GIP-a za procjenu pridržavanja bezglutenske prehrane (GFD), njihova povezanost s tTG-om nije u potpunosti razjašnjena. Bolje razumi- jevanje ovog odnosa moglo bi unaprijediti praćenje bolesnika s celijakijom.
Cilj. Procijeniti koncentracije fekalnog GIP-a kod bole- snika s celijakijom, ispitati njihovu povezanost s razi- nama tTG-a te procijeniti korisnost kombiniranja ovih biomarkera za upravljanje celijakijom.
Metode. Ovo presječno istraživanje uključilo je 60 bolesnika s celijakijom na GFD-u i 10 zdravih kontro- la. Koncentracije fekalnih i serumskih GIP-a kvantifi- cirane su testom ELISA, dok su razine tTG-a mjerene serološki. Za statističke analize primijenjeni su Mann- Whitneyjev U-test za usporedbu skupina te Spearma- nov koeficijent korelacije za procjenu odnosa između biomarkera.
Rezultati. Medijan koncentracije fekalnog GIP-a kod bolesnika s celijakijom bio je značajno niži (39,0 ng/g) u usporedbi s kontrolnom skupinom (474,2 ng/g; p<0,001), što potvrđuje pridržavanje BGP-a. Slično tome, serumski GIP bio je niži kod bolesnika s celija- kijom (p<0,001). Nije utvrđena značajna povezanost između razina GIP-a i tTG-a (ρ=0,114, p=0,387), što
ukazuje na to da mjere različite aspekte aktivnosti bolesti.
Zaključak. Ova studija posebno je procjenjivala kon- centracije fekalnog GIP-a u bolesnika s celijakijom i njihovu korelaciju s razinama tTG-a. Naši rezultati ne pokazuju značajnu korelaciju, što ukazuje na to da ovi biomarkeri procjenjuju različite aspekte aktivnosti bolesti. Studija potvrđuje osjetljivu prirodu GIP-a za detekciju unosa glutena i ulogu tTG-a u reflektiranju imunosnog odgovora i oštećenja sluznice. Integrirana upotreba ovih biomarkera može poboljšati upravljanje i praćenje celijakije, omogućujući precizniju procjenu pridržavanja prehrane i imunosne aktivnosti.
![]()
Ključne riječi: celijakija, bezglutenska prehrana, transglu- taminaze
![]()